#010: Frequency Therapeutics Deep Dive.

In this edition of Longevity Marketcap Telemetry:
- Notable Last Week
- Longevity Futures
- Frequency Therapeutics Deep Dive
- Technical Analysis
Notable Last Week
Investing
- Frequency Therapeutics shows positive FX-322 Phase 2a data for sustained improvement in hearing loss. This was a huge win for the Progenitor Cell Activation (PCA) drug developer and regenerative medicine company. The stock was up 32% on the week following the news. For more analysis read on.
- resTORbio and Adicet merger. resTORbio, the anti-aging rapalog drug development company, finally merged with Adicet Bio — a CAR-T cancer therapy biotech. The newly merged company now trades on the Nasdaq with the ticker ACET.
As part of the deal, Adicet shareholders own 75% of the company. resTORbio shareholders own the remaining 25% and also exclusive benefit from any 3rd party commercialization of the RTB101 drug.
This move was not a great development for the longevity industry since the new company no longer has anti-aging as its main focus. But given the high profile Phase 3 failure of RTB101 for treating respiratory illness last year perhaps the company was on its last legs anyways. - Juvenescence to go public in 6 months. Jim Mellon, billionaire patron saint of longevity investing and co-founder of Juvenescence, was featured on a Master Investor webinar last week. In the webinar he let slip that he expects Juvenescence to go public within 6 months. This timeline is much more aggressive than the 1.5 years he mentioned at ARDD 2020. I imagine he senses what all other IPO-hopeful companies are feeling now: The market is hot for IPOs and they should take advantage of the froth while it lasts.
- Added ChromaDex to LongevityMarketcap.com. ChromaDex (CDXC) is a manufacturer of a popular nicotinamide riboside “longevity” supplement that is believed to raise NAD+ levels. I resisted adding ChromaDex since I am skeptical of all supplements or any product that is sold before the science is solid. However there’s a lot of research being done on NAD+ boosters so I will list them for the time being.
- Aging Research and Drug Design 2020 Conference videos available online. I recommend watching the VC panel and Jim Mellon’s talk.
Trading
- Unity Biotechnology shows some life. An extremely disappointing failure of their UBX0101 senolytic drug to treat osteoarthritis of the knee caused the stock to crash nearly 66% in August. However, after consolidating at around $3 / share the stock bounced last week — up 30%.
Reasons for this surge: Unity announces that it will cut 30% of its workforce in order to focus on their ophthalmology program. There was also a big jump in price on Friday when Unity Software (U), popular game development company, began trading. Perhaps these were error buys? If you don’t believe people could be so stupid I would direct your attention to the charts of ZM and ZOOM. - XBI Biotech Index ETF: +11% on the week. Nasdaq: -2.6%. SPX: -0.6%. Biotech had seen nearly 6 weeks of straight declines so the bounce last week not surprising. But the Nasdaq is still falling, blowing through the 50 day SMA without much relief. If things don’t turn around by the week of October 5th I would be more confident that this is no longer a brief “1 to 4” candle correction on the weekly SPX chart.
Longevity Futures
Investing
- Deep Longevity’s Young.ai app launch coming September 29th for iPhone. Want to use multifactorial deep aging clocks to measure the rate of your biological aging. Well, there’s an app for that.
- Longevity Investors Conference. October 1st, 2020. 12:05 PM CET. First two talks are free. The rest of the day is paid ($395). Lots of great speakers: Jim Mellon, Aubrey de Grey, David Sinclair, Nir Barzilai, Dr. Joon Yun, Eric Verdin, and many more. Register in advance
- Eurosymposium for Healthy Ageing. October 1 – 3, 2020. Free, but register in advance. Research focussed conference. Speakers include: Aubrey de Grey, Alex Zhavoronkov, Josh Mitteldorf, Irina Conboy, Greg Fahy, Nir Barzilai, and more.
Trading
- Frequency Therapeutics (FREQ) and Denali Therapeutics (DNLI) are close to their 52 week highs. See Technical Analysis for more.
- Computational chemistry and drug discovery company Schrodinger (SDGR) fell nearly 50% in the past 2 months. Momentum indicators are showing potential for reversal. I’m interested.
Frequency Therapeutics Deep Dive
First, watch this video:
In my newsletter two weeks ago, I decided to do a brief survey of each of the 16 longevity biotech companies listed on LongevityMarketcap.com.
One of the companies that surprised me the most was Frequency Therapeutics. When I first added the company, I thought it was solely focussed on restoring hearing loss — an important problem for aging populations.
But the company actually has grander regenerative medicine ambitions with their novel approach to stimulating progenitor cells all over the body using locally administered small molecule drugs (huge advantage). They are currently in the discovery phase for extending their technology for a Multiple Sclerosis treatment that regenerates the neuronal myelin.
I’m in. But what about position size?
Frequency’s positive FX-322 Phase 2a clinical trial data showing sustained improvements in hearing and subsequent 30% rise in share price last week was compelling. So I ended up making a small investment in the company in order to quell any FOMO welling up in my gut.
I think Frequency has a lot of potential.
Should I increase my position in FREQ? This calls for some due diligence. My approach for this is simple: Identify the existential risks to the business and if no red flags show up make an appropriately sized position given the company’s market cap and personal portfolio risk tolerance.
So what could kill my investment thesis in Frequency Therapeutics?
Does the science come from a reputable history of results?
- The scientific co-founders include Robert Langer (MIT) and Jeff Karp (Harvard, MIT, BYU).
- Robert Langer is a LEGEND in biotech and medicine. His Wikipedia page will make you feel like something the cat drug in. He has co-founded dozens of biotech companies (including Moderna — hot COVID-19 vaccine stock), is the 8th most cited person in the world, and has over 220 major awards for his work.
- It’s fairly clear that the scientific basis for the company comes from a good lineage. Papers and awards can always be retracted. But the dozens of successful biotech companies launched from Langer’s lab increases my confidence tremendously.
What was the foundational science? Has any of the science been replicated?
- Langer and Karp showed that they could robustly regenerate hair cells in the cochleas (inner ear organ) removed from mice, non-human primates, and humans placed in culture. The death of these hair cells in the inner ear are the cause for age-related / progressive noise-induced hearing loss. These cells do not regenerate naturally.
- The foundational work started with Langer and Karp’s investigation of how intestinal cells regenerate themselves. They found certain epithelial intestinal progenitor cells (a more differentiated stem cells) expressed a surface protein called Lgr5+ and determined the signalling pathways that caused them to divide. This same protein was found in the progenitor cells that differentiate into hair cells in the cochlea.
- Langer and Karp were able to find a mix small molecule drugs that activated pathways (Wnt and Notch) that could cause the Lgr5+ progenitor hair cells to divide and differentiate into mature hair cells.
- The key drugs used were CHIR99021 (a GSK3 inhibitor) and valproic acid (a histone deacetylase inhibitor —epigenetically modulates gene expression). They are the basis for FX-322, Frequency’s hearing loss therapeutic being tested in clinical trials. (Also some added gels and whistles for in-vivo drug delivery to the inner ear)
- Their technique was able to produce a 2000-fold expansion in the number of progenitor hair cells in explanted mammalian cochlea. The differentiated hair cells possessed molecular machinery necessary for function.
- The technique has been replicated in labs associated with the original work (at least in mice). The only other work that seemed to replicate their hair cell regeneration technique was this one. It should be noted that Karp and Langer’s work was published in in 2017, — only 3ish years ago.
Do they have IP protection?
- Here are Frequency Therapeutics’ patents.
- The most relevant patent involves the use of GSK3 inhibitors for controlled proliferation of progenitor stem cells
- I’m not a patent lawyer so I cannot comment on the strength of this patent. I only know that the patent exists.
Is the management team experienced and capable?
- Who is Frequency Therapeutics (operations, management)? These are the people tasked with getting the company to the “light switch moment” Phase 3 readouts.
- CEO, David Lucchino: Frequency is his third company. Previously he co-founded Semprus Biosciences (also with Robert Langer) and was also CEO of EntregaBio. Semprus was acquired by Teleflex for $80 million. EntregaBio seems like it flopped.
- CSO, Chris Loose PhD: Also a member of the Robert Langer mafia. Worked as CTO at Semprus and previously a chemical engineer at Merck.
- Chief Development Officer, Carl LeBel, PhD: Was Chief Scientific Officer at Otonomy, Inc. (an inner ear biotech), President and CEO of Akesis Pharmaceuticals, Inc, and spent more than 14 years at Amgen as an executive director.
Do they have enough cash to get to Phase III?
- According to Frequency Therapeutics’ last business update in August and 2nd Quarter financial results, the company had $195.4 million in cash as of June 30, 2020.
- They project their cash runway will take them to 2023 at least. This estimate does not include revenue milestones from their ex-USA licensing agreement with Astellas.
- Frequency was also able to raise an additional $40 million in cash from a private placement from VCs and investment firms in July 2020.
- Frequency burned ~ $28 million in R&D and administrative costs over the last 6 months.
- In an interview with Carl LeBel, Chief Development Officer of Frequency Therapeutics, he expects that the Phase III will be shorter compared to other drug trials (such as cardiovascular drug trials that take 5 years or more). LeBel believes they will only need 100s of patients because of the reduced placebo effect when dealing with word recognition audio tests (etc). He also feels they can get the treatment to market within this decade, at the very latest.
- FX-322’s Phase 2 is still underway and they are still enrolling patients. They expect to report the study data (interim?) in Q2 of 2021.
- Phase 3 usually has more patients. This should mean more cost. I imagine the company will need more a little more cash to get to the end of Phase 3 which could last as long as 3 – 5 years. If the rest of the Phase 2 data is good they should have no problem raising that money.
What is the business model? What is their Total Addressable Market? What is a reasonable valuation for the company?
- There are no clinically approved therapeutics for hearing loss.
- The global hearing aid market is projected to reach ~$8 billion by 2023 with a CAGR of ~ 6%.
- The hearing aid market underestimates the total addressable market for hearing loss as wear rates are very low. An estimated 80% of people who could benefit from wearing hearing aids do not wear them, mainly because the perceived benefit of wearing the hearing aid does not out weigh the negatives (handling, comfort, noise, stigma and appearance, cost, maintenance)
- The hearing loss market could be 5x bigger than the hearing aid market, and is only getting bigger as demographics in wealthy countries skew older.
- A single dose Frequency Therapeutics treatment would address most if not all of the negatives of using hearing aids to treat hearing loss.
- Let’s say Frequency Therapeutics is able to get their drug to market by 2025 and captures an ultraconservative 5% of the hearing loss market. This would translate to yearly revenues of ~ $2 billion.
- Frequency’s FX-322 is a small molecule drug so it is easy to manufacture — low COGs compared to biologics or gene therapy. It does require an injection into the inner ear, though. I imagine profit margins will be reasonable. Let’s estimate annual profits at ~ $0.7 billion — roughly 33% margin.
- The patent on FX-322 will expire after 20 years. But let’s say some new technology (bio or otherwise) displaces FX-322 within 10 years, a conservative guess.
- Assuming the yearly growth in FX-322 profits (remember hearing aid market CAGR was estimated to be 6%) at least matches the risk free rare of return (not going to speculate what that is) then total net present value of the company’s profits is ~$7 billion, which would be the company valuation. This would represent a 8.5x return from the company’s current valuation at $816 million.
- Notes: Frequency Therapeutics has a licensing agreement with Astella’s who will commercialize the drug outside of the USA. Frequency has received revenue from this deal and will also receive future royalties. But the cash from the Astella’s deal acquired in the present to de-risk will cut into Frequency’s full potential profits.
- Notes 2: I only factored in potential profits from Frequency’s hearing loss pipeline. They also have a pipeline for Multiple Sclerosis. This pipeline seems much riskier and CEO David Lucchino mentioned they would need outsider investors to bring it to clinical trials. If they roll the dice on a MS trial and it fails it could be dilutive to the share value of the company.
- Notes 3: Another possible market for FX-322 is sufferers of tinnitus. There is a passionate and hopeful tinnitus community on reddit that is following Frequency’s trials religiously.
Who are their competitors?
- Frequency Therapeutics is the first and only company in the world to demonstrate a clinical improvement in hearing loss.
- Other biotechnology companies working on hearing loss: Otonomy (OTIC), Auris Medical, Novartis (NASDAQ:NVS).
- Other competitors could include advanced wearable hearing devices or Neuralink-like technologies that more seamlessly improve hearing. Also improved cochlear implants or synthetic inner ear organs.
Was the recent Phase 2a data that great? What’s the catch?
- Frequency reported preliminary Phase 2a clinical data that showed sustained and statistically significant improvements in hearing.
- Testing 13 and 21 months after a single injection of FX-322 to the inner ear for 3/5 patients that responded to the drug initially.
- Word recognition scores were up 87% .
- Pure tone detection @8kHz improved by 10 -15 dB for 3/5 patients.
- No improvement in hearing for placebo after 90 days.
- CAVEATS: Small n =5. Not a lot of data to go on. A bigger Phase 2 trial will test 1x 2x and 4x dose.
- CAVEAT 2: Didn’t work on everyone. Why? Need to determine hit rates. Dose might improve this.
- VERDICT: This is an incredible result. Frequency is the first to demonstrate the reversal of hearing loss. EVER. Result looks quite durable for 60% of the patients after a single injection. Amazing, but is it enough? Will people pay for this level of hearing improvement?
What about applying their technology to regenerate other parts of the body? Is it feasible? Do they have the cash?
- In promotional materials, the company’s founding researchers (Langer and Karp) talk about how the progenitor cells they activate can be found in other tissues like the brain and hair follicles. This opens up the possibility for extending their regenerative technology.
- However, the only other treatment in their pipeline is for Multiple Sclerosis (regenerate the myelin in the neurons) which is is discovery phase — not even pre-clinical.
- Although the company is seemingly getting good results in their hearing loss pipeline, the lack of a solid secondary pipeline makes me question how translatable the technology is to other tissues in the body. Perhaps there is a practical reason why they would be more difficult.
- A treatment to regenerate hair follicles (which also have Lgr5 markers) as a treatment for alopecia (male pattern baldness, etc) would certainly be lucrative. They could even grow the follicles ex-vivo to supply donor follicles in hair transplant surgeries. I am very curious why this isn’t being pursued.
Technical Analysis
Mirror, Mirror on the wall
When will J.Pow save us all
This is the section of the newsletter where we let rampant speculation and Nostradamic geometry run wild.
It’s time for technical analysis.
What stocks and assets look ripe for momentum reversal? Let’s see.
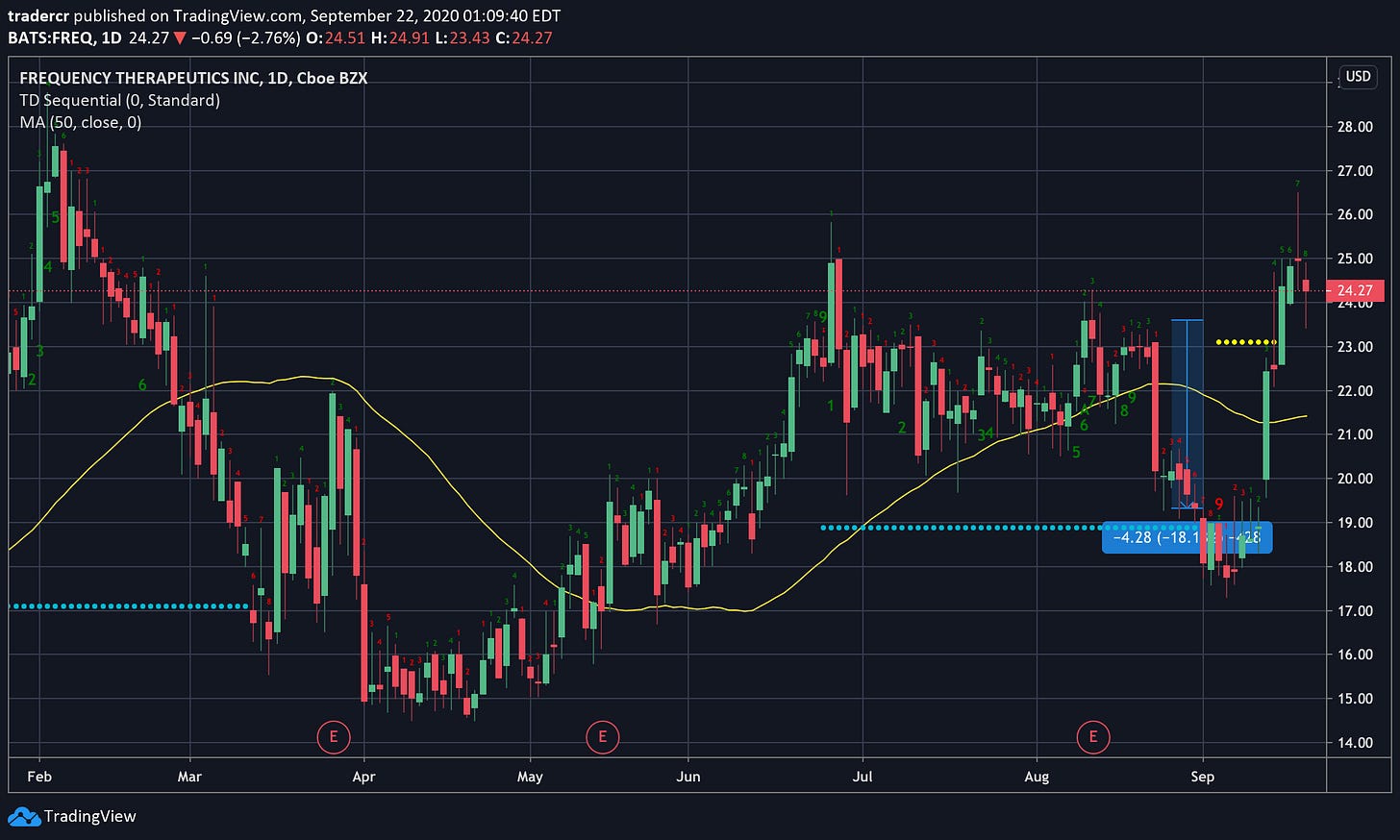
Frequency Therapeutics (FREQ)
Daily chart.
In newsletter #007, I wrote how FREQ was getting close to a TD 9 Buy signal and I was looking to buy. The signal came. And it turns out that was a great time to buy since the stock was up 30% only days later (on positive Phase 2 trial data — of course TA does not predict this stuff).
I didn’t buy at that time because I was too preoccupied with the blow up in volatility in the markets. Darn.
I eventually did buy and after researching for this week’s newsletter, I feel much more confident in adding to my position. But when?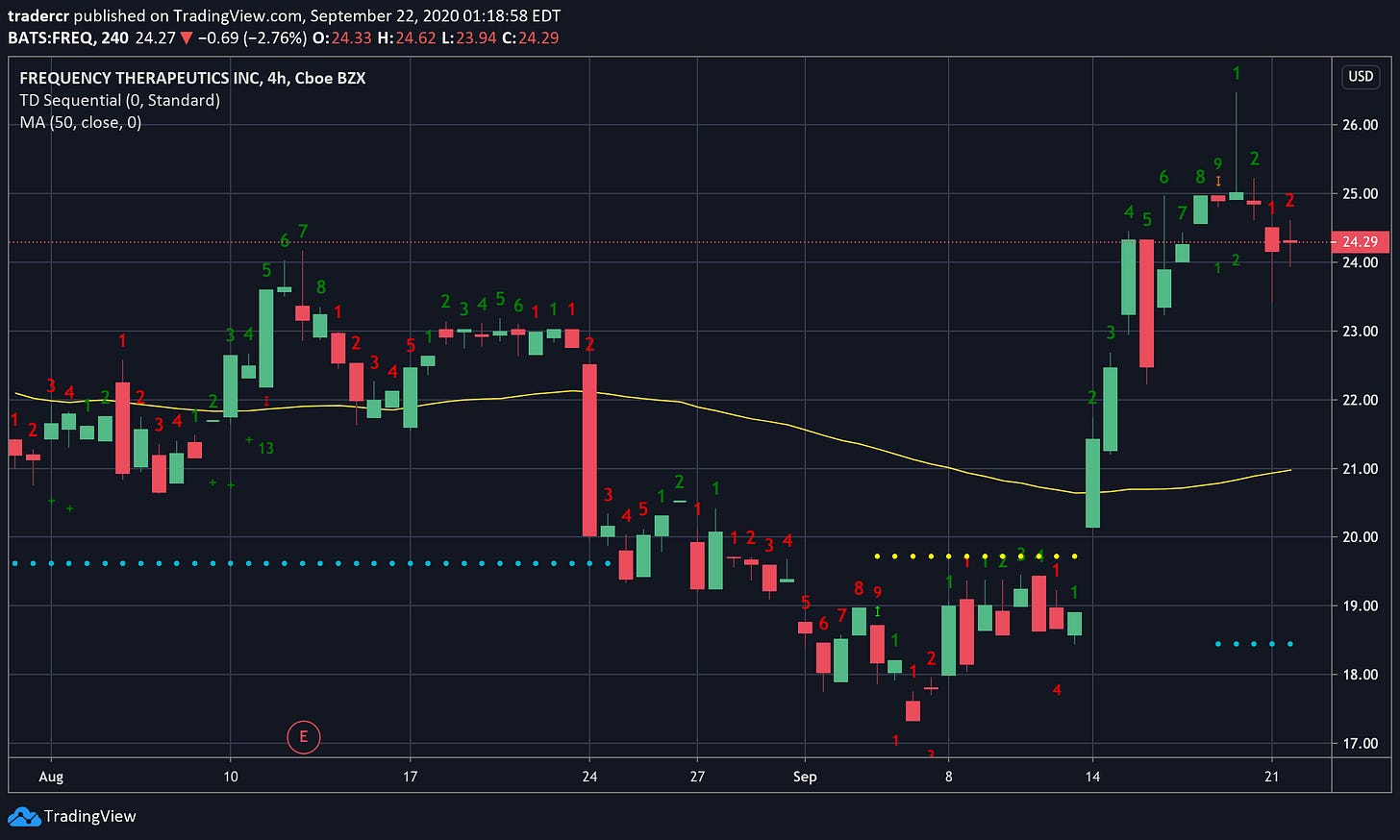
4 hr chart.
If we look at the momentum indicators it looks like we might already be in a correction. The 4-hour chart (good for trading during sharp transitions like clinical trial announcements) called the previous two turning points rather beautifully so I will be watching this timescale carefully. If we begin to trend up again by the Wednesday then the correction (on the 4 hr timescale) might be over and I may consider entering.
If I miss a good entry and the stock takes off, I will probably just buy when it breaks it’s all time high around ~$28. I think this stock could easily go to $100 if their trials are successful.
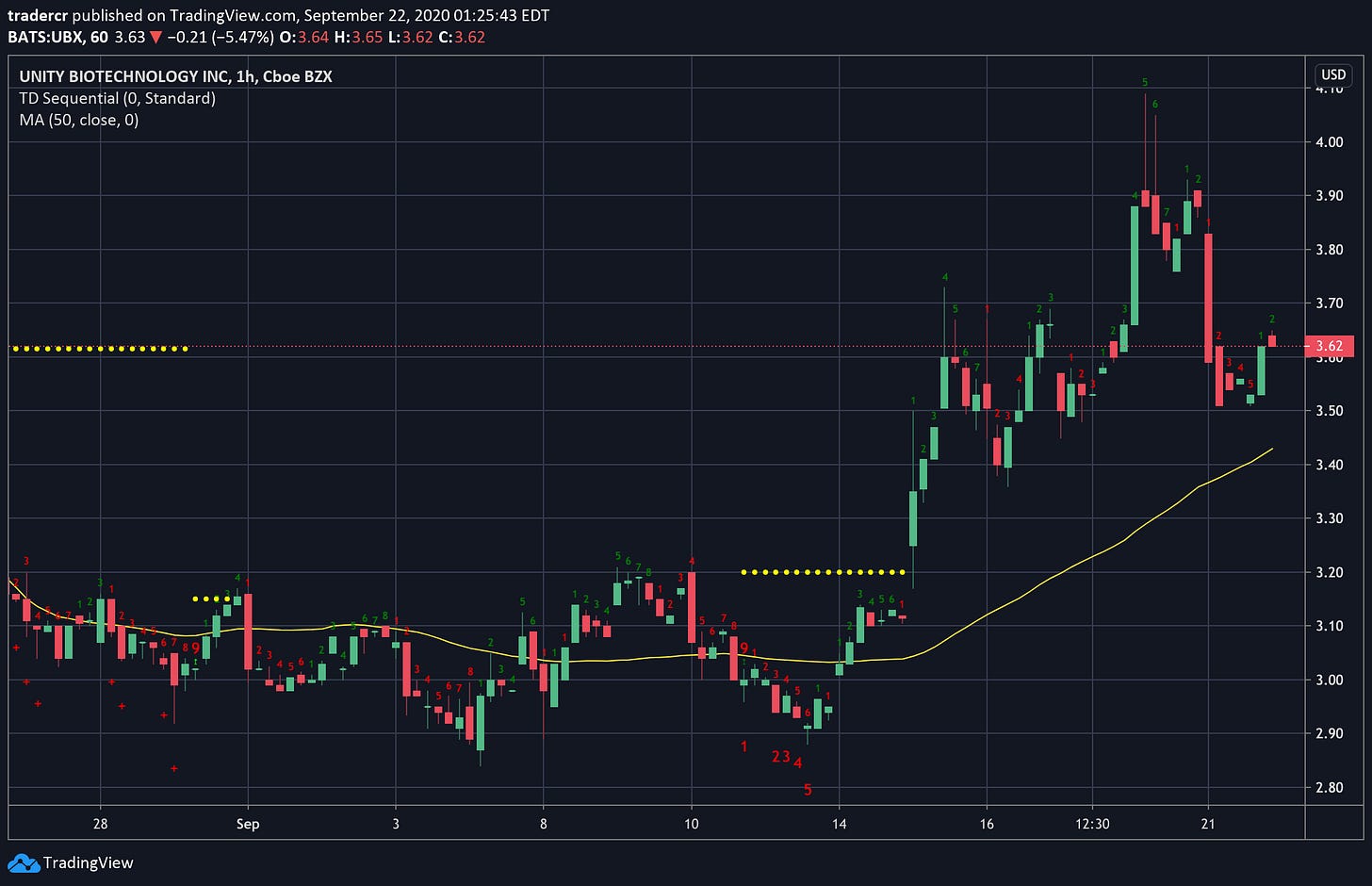
Unity Biotechnology (UBX)
Hourly chart.
After crashing 66% on the news of the failed UBX0101 osteoarthritis trial, the senolytic drug company consolidated around $3 for nearly a month before popping 30% last week on news of a reduction of workforce and thus extension of their cash runway.
This news was all the depressed stock needed to come back to life.
This Monday saw a bit of a pullback. But I like what I see so far. A weekly close above $3.84 would be a bullish signal.
I already probably have too much UBX stock, but at this price the temptation to add to my position is palpable. The only short term risk I see is if something delays their UBX1325 ophthalmology trial. A Covid lockdown (or two) could do that.
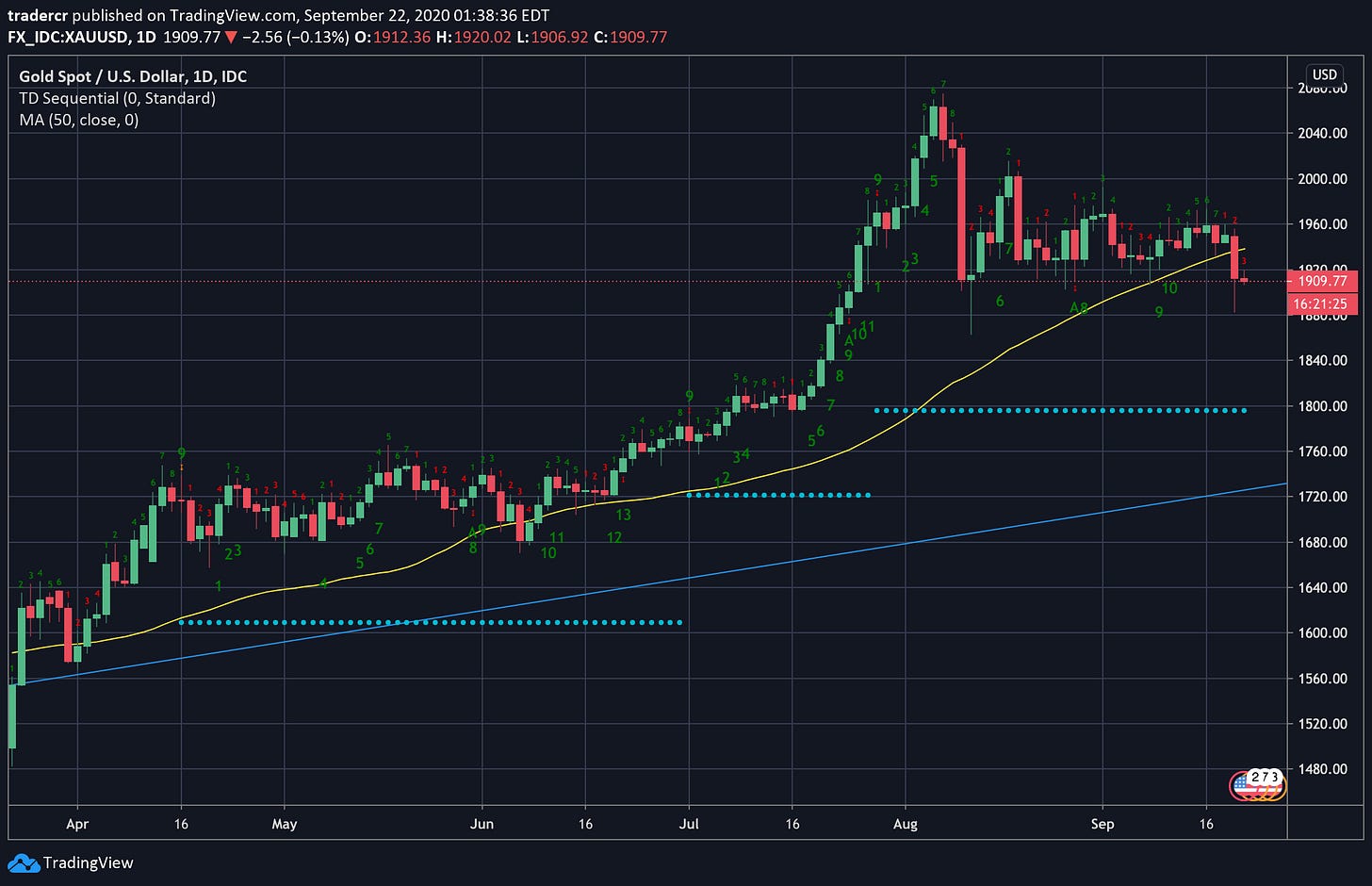
Gold (XAUUSD)
Daily chart.
I wrote in newsletter #008 about Gold and how I expected it to break below $1920.
Finally!
After bouncing several times with lower highs like a bad high school math problem, the descending triangle finally broke, taking out the 50 day SMA with it.
I already trimmed my gold position above $2000 / oz at the end of August when both my mother and sister asked me how to buy gold out of the blue. The TD momentum indicators (daily, weekly, AND monthly) were all screaming 9 Sells, too.
I would like to buy back in at some point. The break of the all time high was notable. Money printing fuels a gilded narrative.
We are way past a simple 1-4 candle correction on the weekly scale so it is now bearish for gold weekly. I’m guessing at most 3 more months of pain before we reverse again. My target is $1775, but might start buying back in at $1800.
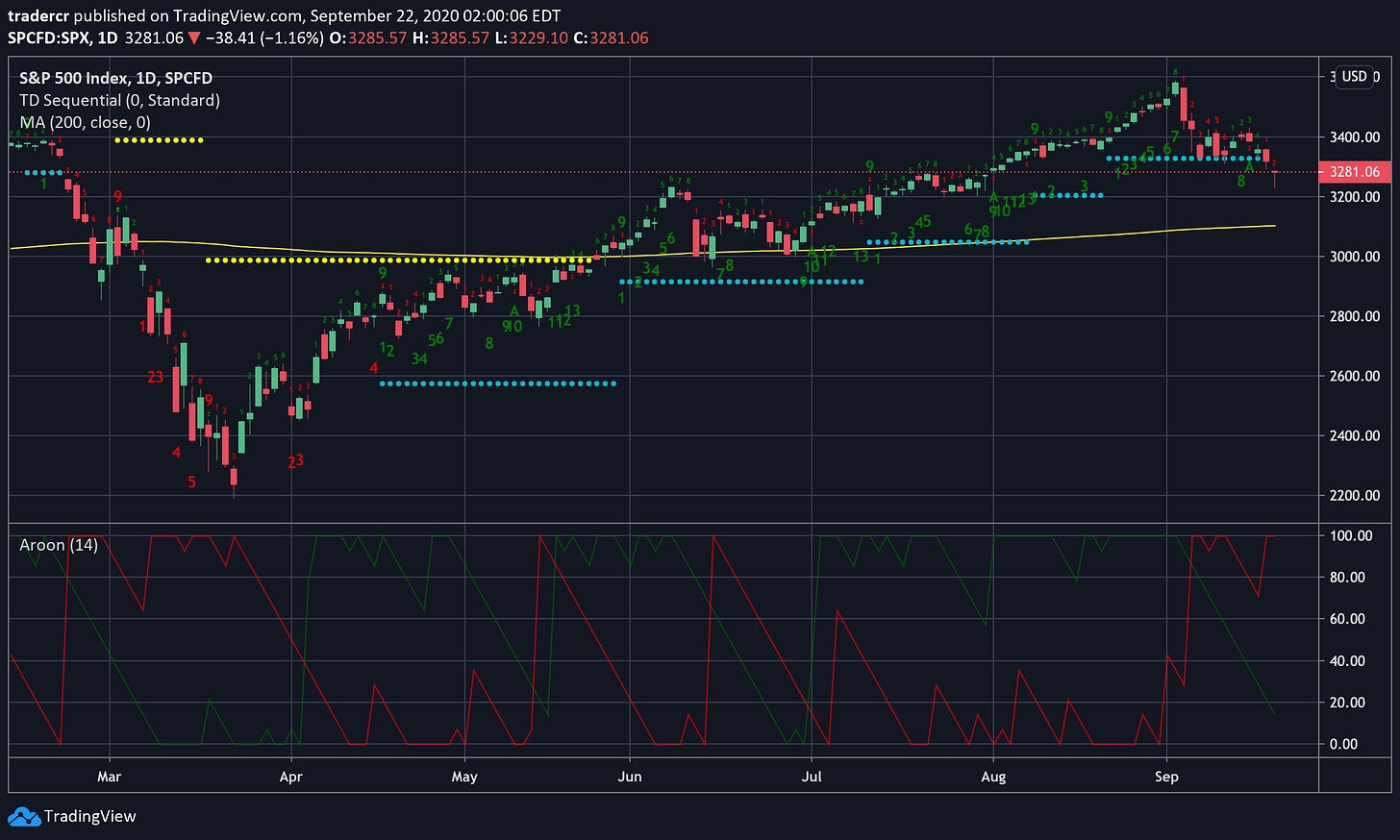
SPX
The SP500 topped on a TD 9 Sell Daily and Weekly two weeks ago and the misery has not subsided. The 200 day SMA has held as support in the last shakeup in June so I expect we will see at least a temporary bounce there. The Aroon indicator is saying stay out of the market right now.
Long term I’m not sure about the market. The 10-year yield is down 10% from its previous swing high but still stuck in a range. Wait and see.